Abstract
CD37 is a tetraspanin plasma membrane protein abundantly expressed on B-cells and represents a promising therapeutic target for the treatment of B-cell malignancies. Although complement-dependent cytotoxicity (CDC) has proven to be a powerful Fc-mediated effector function for killing hematological cancer cells, CD37 antibody-based therapeutics currently in clinical development are poor inducers of CDC. Here we present DuoHexaBody-CD37, a novel humanized IgG1 bispecific antibody targeting two different CD37 epitopes, with an E430G hexamerization-enhancing mutation, for the potential treatment of B-cell malignancies.
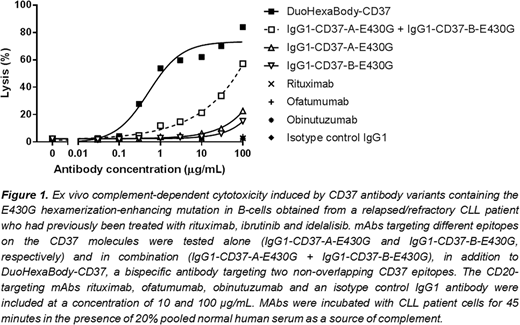
The natural process of antibody hexamer formation through intermolecular Fc-Fc interactions between IgG molecules after cell surface antigen binding can be improved by introducing a single point mutation such as E430G in the IgG Fc domain, thereby facilitating more efficient C1q binding and complement activation (Diebolder et al., Science 2014; de Jong et al., PLoS Biol 2016). The hexamerization-enhancing mutation E430G was introduced into two humanized CD37 monoclonal antibodies (mAbs) that bind non-overlapping CD37 epitopes. Different antibody formats and combinations, including the single antibodies, combinations of the mAbs and bispecific mAbs were tested for their capacity to induce CDC and antibody-dependent cellular cytotoxicity (ADCC). The bispecific hexamerization-enhanced antibody variant DuoHexaBody-CD37, showed superior CDC activity compared to the single hexamerization-enhanced mAbs and the combination thereof, both in vitro over a range of different B-cell lines, and ex vivo in tumor cell samples obtained from patients with chronic lymphocytic leukemia (CLL). In a CDC assay using tumor cells obtained from a relapsed/refractory CLL patient who received prior treatment with rituximab, ibrutinib and idelalisib, DuoHexaBody-CD37 induced almost complete lysis (84% lysis at a concentration 100 µg/mL), thereby outperforming the single HexaBody molecules (15% and 23% lysis) and the combination (57%) (Figure 1). In addition to its potent CDC activity, DuoHexaBody-CD37 was also capable of inducing potent ADCC of Daudi cells (EC50 = 12.3 ± 9.5 ng/mL), as assessed using peripheral blood mononuclear cells from 8 healthy human donors in a standard chromium release assay.
In assays using whole blood from 6 healthy human donors, DuoHexaBody-CD37 showed efficient B-cell binding and potent and specific depletion of the B-cell population (98% ± 1.3% depletion at 10 µg/mL, EC50 = 0.85 ± 0.284 µg/mL). Furthermore, DuoHexaBody-CD37 induced significant inhibition of tumor growth in vivo in Daudi-luc Burkitt's lymphoma and JVM-3 CLL mouse xenograft models, at doses as low as 0.1 and 1 mg/kg (p<0.05), respectively.
In summary, we present a novel therapeutic antibody that, for the first time, combines proprietary DuoBody® and HexaBody® platforms. DuoHexaBody-CD37 induced highly potent CDC and efficient ADCC in preclinical models, suggesting that DuoHexaBody-CD37 may serve as a potential therapeutic mAb for the treatment of human B-cell malignancies.
Oostindie:Genmab: Employment, Equity Ownership. Van Der Horst:Genmab: Research Funding. Overdijk:Genmab: Employment, Equity Ownership. Strumane:Genmab: Employment, Equity Ownership. Verploegen:Genmab: Employment, Equity Ownership. Lindorfer:Genmab: Research Funding. Cook:Genmab: Research Funding. Chamuleau:Gilead: Research Funding; BMS: Research Funding; celgene: Research Funding; Genmab: Research Funding. Mutis:Gilead: Research Funding; Celgene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Research Funding; Novartis: Research Funding; OnkImmune: Research Funding. Schuurman:Genmab: Employment, Other: Warrants. Sasser:Genmab: Employment, Equity Ownership. Taylor:Genmab: Research Funding. Parren:Genmab: Equity Ownership; Lava Therapeutics: Employment. Beurskens:Genmab: Employment, Equity Ownership. Breij:Genmab: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal